Abstract
Bortezomib, thalidomide, and dexamethasone (VTD) combination followed by consolidation with autologous haematopoietic cell transplantation (ASCT) has been an established induction regimen for newly diagnosed multiple myeloma (MM) for many years. Recently, long-term follow-up results of Italian phase 3 trial showed an impressive PFS and OS, respectively of 34% and 60% at 10 year.
Aiming to investigate the real-life experience at our Institution, hereby we report the results of a retrospective analysis including 106 consecutive patients receiving VTD induction followed by single or double ASCT consolidation between 2006 and 2021. Patient characteristics are summarized in Table 1. Median age at the beginning of therapy was 60.3 years (range 35-73), with 59% of males. In line with other reported MM cohorts in terms of disease characteristics and risk factors, extramedullary disease was documented in 12% of patients and high risk cytogenetics in around one third. Median number of VTD cycles prior to ASCT was 4 (range 1-6).
Response after VTD induction was sCR in 19%, CR in 9%, VGPR in 45%, PR in 17%. Stable disease or no response was seen in 10% of cases. Non responding patients were treated with a lenalidomide-based re-induction, all obtaining at least PR. Intermediate-dose cyclophosphamide (3-4 g/m2) + G-SCF was the PBSC mobilizing schema in 98% of patients, with successful harvest in 97%. Median number of CD34+ collected cells was 12.6 × 106/kg, allowing to plan a double ASCT strategy in 92% of patients. Administration of plerixafor was required in 5.8% of patients. A second ASCT was planned in patients with high-risk features and no adverse event grade ≥3 after the first transplant. Eleven patients (10.3%) did not proceed to ASCT due to adverse events or refractory disease. Among autografted patients, 53% received single ASCT and 39% double ASCT. High dose melphalan was the conditioning regimen for all ASCT, with 200 mg/msq in 85% of cases; in unfit patients (15%), the dose was reduced to 140 mg/msq (100 mg/msq in one case). Response after ASCT was sCR in 47% of patients, CR in 20%, VGPR in 25% and PR in 6%.
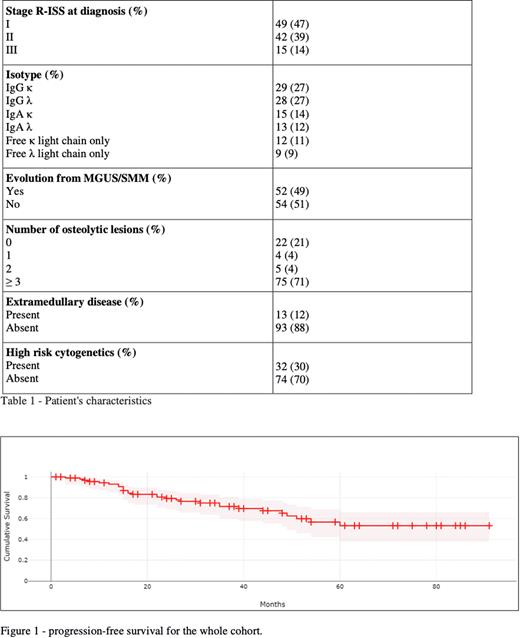
For the whole transplanted group, the 10-year OS and PFS were 77,6% and 53%, respectively (figure 1). After a median follow-up of 53 months (1-187), 89% of patients were alive and 72% were still maintaining response, not requiring any second line treatment. Main cause of death was disease progression. Median PFS and OS were not reached. No significant differences were documented between patients receiving single or double ASCT in terms of OS and PFS. The 5-year OS and PFS in high risk cytogenetics patients were 77% and 36%, compared to 89% and 57%, respectively, in the rest of the cohort. Six patients were diagnosed with a secondary malignancy (3 breast cancers, 1 colon cancer, 1 MDS and 1 oral cavity cancer).
In conclusion, VTD followed by ASCT in real life resulted in excellent and durable responses in untreated and fit MM patients, with the large majority not requiring further therapy after a median follow-up of more than 4 years. The recent addition of daratumumab to the first line regimen is expected to further improve patient outcomes.
Disclosures
Bolli:Janssen: Consultancy, Speakers Bureau; Amgen: Speakers Bureau; Takeda: Honoraria; GSK: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal